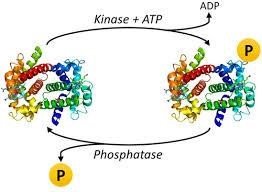
From biochemistry and molecular research, it was found that approximately 30% of proteins in eukaryotic cells are subjected to phosphorylation, and this regulates cellular activities like cell cycle, metabolism, differentiation, and neuronal communications.
Phosphorylation deals with adding chemicals of a phosphoryl group (PO3) to organic molecules. It is carried out through enzymes such as kinases, phosphotransferases, etc. Phosphorylation is important as it is a key reaction in protein, enzymes, sugar metabolism, releasing and storage of energy. In cells, phosphorylation performs a crucial role in glycolysis, protein-protein interaction, protein degradation, enzymes inhibition regulations, and maintenance of homeostasis. Among the different types of phosphorylation is protein phosphorylation.
What is Protein Phosphorylation?
Protein phosphorylation occurs when the phosphoryl group is added to an amino acid (serine). Protein phosphorylation is key to different physiological processes that control the cardiovascular, gastrointestinal, immune systems, mechanism, behavior and neurological actions, musculoskeletal and endocrine systems. It also contributes to pathological conditions like cancer.
In the global control of DNA replication during cell cycles and stress-induced replication block mechanisms, protein phosphorylation also occurs. It serves as an extraordinary life component process that includes signal transduction pathways that underlie cellular proliferation, metabolism, differentiation, survival, mobility, and gene transcription. Phosphorylation of histones is an example of protein phosphorylation.
When accessing protein phosphorylation, the methods may vary, considering different factors such as the question being asked and reagents or availability of specialized apparatus.
Also read: 7 Reasons to Get Scheduling Software for Your School
Methods for detecting protein phosphorylation
Several methodologies are in use in detecting protein phosphorylation in biochemistry or molecular biology.
Kinase Activity Assays
Protein kinases are common elements in multiple signaling networks that influence several downstream effectors accountable to biological responses. Kinase activity is determined within vitro kinase activity assays. Then, a purified kinase, an unphosphorylated substrate, and radiolabelled ATP are mixed. The substrate is further analyzed for phosphorylation using the radiolabelled phosphoryl group. However, other in vitro kinase assays do not make use of radiolabelled ATP but makes use of detection antibody like the MMP2 antibody, which is specific to ADP instead.
Drug developers employ this method to identify new kinases substrates or screen small-molecule kinases inhibitors. This research method is high throughput screening, and it is excellent for drug discovery and screening. However, little is revealed using the kinase activity assays about the protein modification. The in vitro results rarely represent the in vivo, nor do they address the role of potential endogenous phosphate activity.
Western Blot

This is the most common and qualitative method for detecting the protein’s phosphorylation state. After the separation of proteins using SDS-PAGE, the proteins are transferred to a membrane from the gel state through voltage. A phospho-specific antibody of interest is added to the membrane, and the phosphorylation antibody is bound by a secondary antibody that is conjugated horseradish peroxidase (HRP). The typical western blot protocol can eliminate the hazards and waste disposals requirements associated with radioisotopes. The sensitivity and specificity of the western blot method depend on the antibodies that are used, like perk antibody. Using western blot for detecting protein phosphorylation requires moderate skills and simple data interpretation. However, it has a low throughput, and it depends more on antibodies.
Flow cytometry

This method is liquid-based, and single cells are characterized using fluorescently-conjugated antibodies, which targets specific protein of interest. When accessing multiple proteins in the cells, filter sets and fluorochromes with non-overlapping spectra must be chosen with diligence. The main use of flow cytometry is in detecting cell surface proteins and does not involve the physical separation of cells. With this, a small and rare population of cells can be analyzed without cell loss or alteration in protein expression.
Though, intracellular proteins like the phosphorylated proteins can also be analyzed through this method. Flow cytometry allows for rapid, qualitative, and single-cell analysis. And it demands high affinity, high specificity antibodies such as the RAD51 antibody, blocking steps, control, and antibodies titration. This eliminates any form of ambiguity in the results due to non-specific binding. Detecting protein phosphorylation using the flow cytometry method demands that the protein be stable and accessible to the antibody used.
Conclusion
To understand the signaling of the cells and phenotype, detecting protein phosphorylation is highly essential. The methods to use depend on various factors such as the objective of the project, expertise, availability, access to apparatus, and the budget involved. Three out of the protein phosphorylation detecting methods are only discussed here. Other methods include SDS-PAGE, ELISA, Phospho-Specific Antibody Development, Mass Spectrometry, Multi-Analyte Profiling, and antibody array. Each of these methods performs better in a different context of use, and the method that fits adequately to the experimental design should be used. The methods of detection that have been discussed here require phospho-specific antibodies or antibodies during sample processing.